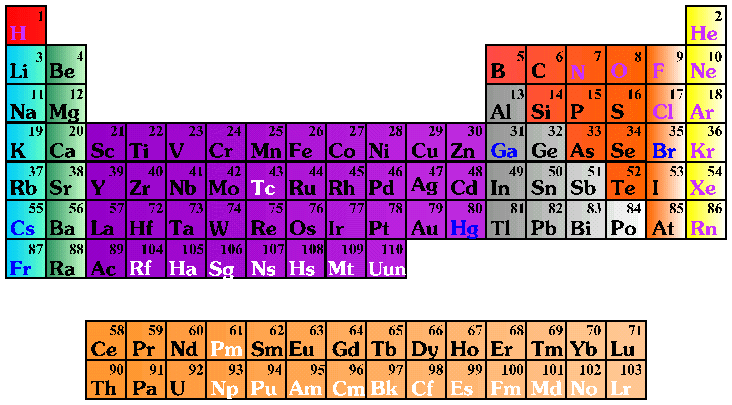
He is very proud of being the
element #16 in the PeriodicTable.
He is very proud of his
16 protons, 16 electrons, and
16 neutrons.
Of his 3 levels of energy, of being a non-metal
and, due to his 6 valence electrons,
of his placement in group VI A.
He is also happy with his atomic mass of 32.06 and
with his four isotopes (32-S, 33-S, 34-S, and 36-S).